There are six National Centers for Advanced Medical Specialties (National Center: NC) in Japan, each of which has its own biobank.
The NC biobanks collect samples and clinical information on diseases in their areas of expertise.
The National Center Biobank Network (NCBN) is a joint project among the six National Centers with the goal of creating a research platform for "new medicines".
To achieve this goal, the six NC biobanks provide their samples and clinical information for medical research and drug discovery.
- Introduction to NCBN by video -
-
Drug development research through industry-academia-government collaboration using an integrated database of disease-specific information based on the NCBN.
AMED Funding for Research to Expedite Effective drug discovery by Government, Academia and Private partnership (GAPFREE) is a program for supporting the development and commercialization of pharmaceuticals in Japan. It involves conducting multilayered omics analysis of genomes, transcriptomes, proteomes, etc., on samples with high-quality clinical information held in the NCBN in collaboration with seven member companies of the JPMA.
The results are combined with clinical laboratory values, image data, and other clinical information to create an integrated database of disease-specific information in a joint industry-academia-government project. Utilizing this integrated database of disease-specific information, we aim to deepen our understanding of diseases. Academia will propose new disease classifications and early diagnosis methods, while the pharmaceutical industry will identify target molecules and biomarkers for stratified patient populations. -
Creation of healthy group and disease control group data necessary for the promotion of genomic medicine for cancer and intractable diseases
Whole genome analyses will be performed for intractable diseases and cancer diseases. Whole genome analyses of healthy control data are necessary for genome analyses for these diseases. The whole genome analyses will be performed for healthy controls or controls for cancer or intractable diseases on samples selected from the 6 NC biobanks in consideration of regional characteristics, and a genome database for healthy controls and disease controls will be created.
-
Genomic analysis for patients with a history of allergies to NSAIDs, antibiotics, contrast media, and alcohol disinfection
This will involve extracting drug allergy history from clinical information held by each NC in the NCBN catalog database. Genome-wide SNP arrays will be performed on drug strains for which more than a certain number of genes are detected, to obtain gene regions potentially causing hypersensitivity to specific drug strains.
-
Genomic analysis for patients with contrast media nephropathy
While predicting the onset of contrast nephropathy is difficult, it is an adverse effect that always needs to be taken into consideration in clinical practice. In this study, we will conduct a genomic analysis of patients with or without contrast agent nephropathy. This will be done by matching information in the central biobank catalog database with electronic patient information in each NC that can be linked to the database via the NCBN, and narrowing it down.
-
Study on the provision of biobank samples with genetic analysis and other data.
This will entail conducting a study on the provision of disease biobank samples stored in the 6 NC biobanks with attached genetic analysis and other data, identifying the issues and establishing a system and structure. The analyzed genomic data will be returned as basic information on individual biobank cases, aiming to improve the quality of the NC Biobanks overall, a data sharing network environment among the 6 NC biobanks will be established and data shared within the NC Biobanks.
-
Genome Research Platform Utilization System Project
The NCBN has been participating in AMED's "Genome Research Platform Utilization System Project" with Tohoku Medical Megabank (ToMMo) and Biobank Japan (BBJ) since FY 2018, and the biobank cross search system started in October 2019. NCBN's bioresources are rich in variety, are subject to high quality control at the ISO9001 level, and can provide detailed clinical information, making them highly valued by researchers in Japan and overseas. The company has been attracting attention .
Biobank cross search system>> AMED BIOBANK CROSS-SEARCH -
Creating a common platform for promoting medical research and development
The Central Biobank will be able to provide NCBN bioresources for the purpose of promoting medical research and development in Japan, and a common platform will be created for this. The catalog database will be part of the common platform for information retrieval among the 6 NC biobanks.
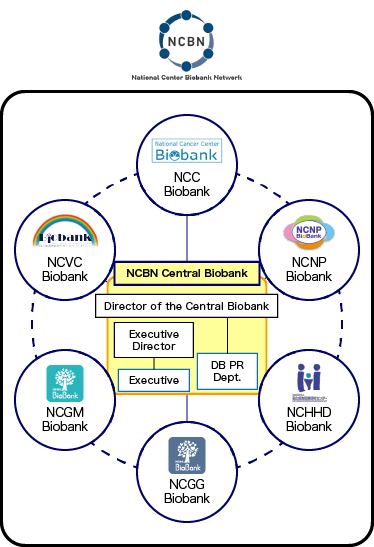
Director of the NCBN Central Biobank
Katsushi Tokunaga

-
Director of the Central Biobank
Katsushi Tokunaga

-
Executive Director of the Central Biobank
Eisei Noiri
-
Executive of
the Central Biobank- Yosuke Omae
- Akino Takase
-
Central Database
Public Relations Department- Koji Kitajima
- Hideyuki Shimanuki
-
Advisor of Biobank
- Yuichi Goto
-
Japan Institute for Health Security
-
Central Biobank related sites







